How Crude Oil is Separated Into Fractions
November 28, 2018
0
If you’re an on-site machinist, you may be expected to work across a variety of industries – often carrying out in-situ jobs such as flange facing, hot tapping or pipe cutting. It goes without saying that the most important thing is to get the job done efficiently and effectively. But if you can demonstrate your knowledge of what goes on at the plant then your credibility can be enhanced – potentially leading to more work in the future.
Oil refining plants create a huge amount of on-site machining work, so having a grasp of the main process involved will put you in a good position. The graphic we’ve created for you below shows the principle behind a fractionating column used to refine crude oil.
Fractional distillation process is the separation of a mixture into its component parts, (fractions).
When heat is applied to the crude oil chemical compounds are separated – causing the fractions of the mixture to vaporize and distil.
View the image below, or to keep a copy, download a copy in PDF format.
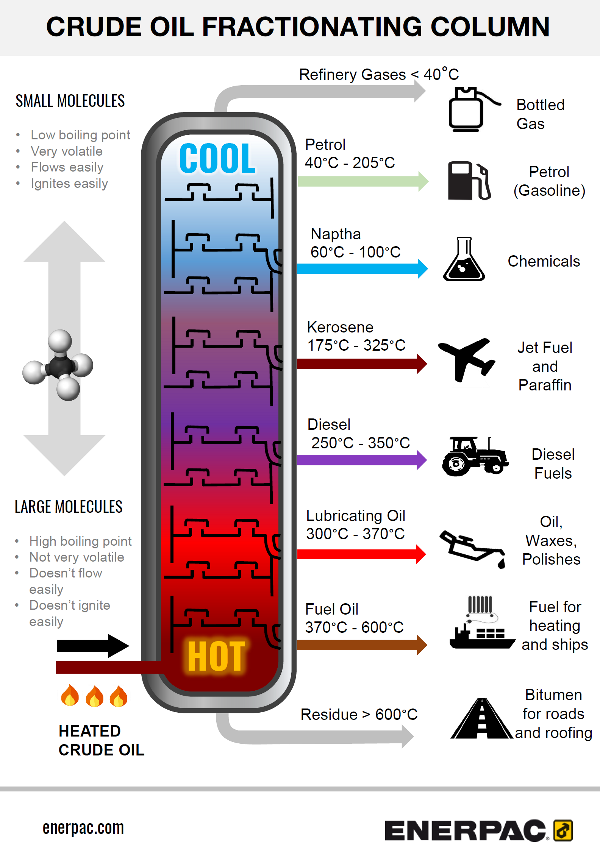
Simple overview of the fractionating process
- Crude oil is heated to around 600 degrees Celsius (1112 Fahrenheit).
It boils and forms vapour. - The vapour enters the bottom of the fractional distillation column
- As the vapour rises it becomes cooler
- The distillation column is filled with trays or plates which collect the liquids as the vapour condenses
- The trays collect the various liquid fractions, which then follow pipework outside the column.
- The collected liquid fractions may pass to condensers, which cool them further, and then go to storage tanks, or to other areas for further chemical processing.
For details of portable machine tools for use in the petrochemicals sector please visit the Enerpac website here